You dont say which sample weighed 770 gms per litre. A litre of pure gasoline weighs 740, kerosene 780 and diesel 840. So a sample weighing 770 will be mostly lighter fractions, gasoline and kerosene with very little diesel. To produce a heavier diesel type fuel it might be better to crack at a higher temp, say 400, and reduce the waxing by adding a pour point depressant. What do you think.
Announcement
Collapse
No announcement yet.
How to turn plastic waste into diesel fuel cheaply
Collapse
X
-
So I must increase the Coils right ...Originally posted by imakebiodiesel View PostYou dont say which sample weighed 770 gms per litre. A litre of pure gasoline weighs 740, kerosene 780 and diesel 840. So a sample weighing 770 will be mostly lighter fractions, gasoline and kerosene with very little diesel. To produce a heavier diesel type fuel it might be better to crack at a higher temp, say 400, and reduce the waxing by adding a pour point depressant. What do you think.
I reside in India ..
we have 230v supply ... per phase
So hw many coils do u think that i can put in parallel .. and get some good temperature !!
Comment
-
Originally posted by aby2maria View PostSo I must increase the Coils right ...
I reside in India ..
we have 230v supply ... per phase
So hw many coils do u think that i can put in parallel .. and get some good temperature !!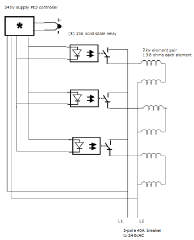
This is the setup I built. The coils are wound on a piece of 1/2" steel tube, about 14" long. I placed 160 turns of 20-gauge nichrome-60 wire on each coil.
Two coils are in series for each circuit. Each element is 13.8 ohms, so each circuit is 27.6 ohms.
Current = 240V / 27.6 ohms = 8.6A
Power = current * current * resistance = 8.6 * 8.6 * 27.6 = 2041 watts or
2kw per circuit.
I have three circuits, or 2kw * 3 = 6kw heating power.
My tank is tall 100# propane cylinder, 15" diameter, about 44" tall.
My tank sits inside a steel cage, just as Jetijs setup does.
I used a 55 gallon barrel as the container.
Inside are three layers of firebrick, 9" tall, carved to fit the drum inside, bevels, like this:
One PID controller operates all three solid state relays primary side in parallel.
If your tank is not as tall, then just place one or two pairs of elements, instead of three.
The PID controller turns the elements on, when the PID set temperature is greater than the temperature reported by the thermocouple. The PID controller turns the elements off, when the PID set temperature is less than the temperature reported by the thermocouple.
You could use a ceramic band drum heater, as an alternative to carving up all the bricks... but you definitely need to apply rock wool insulator to conserve heat.
Comment
-
reactor heating vessels and clays
here are some ideas for people starting from scratch and might want to consider propane power - and it might be possible to also circulate some of the non-condensiable gases to the burner as well.
here are some examples of some very expensive units that could be used as reactor heaters- they are like 4000-5000$ US ( too much ) they have electric as well
gas fired soft metal melters
Wenesco's Gas-Fired Melters
i am taking inspiration from the gas fired units and I am building one ( almost done ) using this 75,000 - 150,000 BTUH propane burner.
see site has a simple design for firing clay:
How to build a raku kiln
i am using a shell from a turkey cooker adding insulation and the 75000 BTU burner and making the reactor out a a large 10" sch 40 pipe with a flange lid on top.
jetijs / asad - hello - they clay shop happens to have many clays including Bentonite clay ( which is in a powder form ) is this what you are using ? a powder?
they have many others as well that sound very catalyst sounding:
Alumina Hydrate - Al2O3
Alumina Oxide - Al2O3
Carbondale Red Clay (C-Red)
Cerium Oxide - CeO2
see this page :
Raw Materials, Stains, Dry Clay & Glaze Ingredients
would you like me test test any of these ? as they are cheap and close to buy for me.
Comment
-
to jetijs
Hello
your study is highly interesting !
please let me construct some comment :
considering your friend so interesting video
‪video‬‏ - YouTube
where we can see the catalyst is kept at high temperature, so the heavy ones would pass it as a gazeous state, without condensing. Allowing the catalyst material to convert many of it into lighter molecules. And only after that, they let the temp going lower and then separate the condensed heavy fractions and let them return to the reactor.
I beleive the catalyst does work at the micropore level. Only a gaz is fluid enough to cross something microporous as a high speed. SO it seems IMPORTANT to have the catalyst free of liquid state product that would clogg it's pores...
please take a look at the chinese seller for catalyst : as you can see, it is only about gaz crossing the catalyst, not liquid. And they add a porous material under it (said breezeblock), so the flux will be regularly managed on the surface, getting maximum catalyst efficiency.
Efficient Waste Oil Refining Catalyst - Detailed info for Efficient Waste Oil Refining Catalyst,waste oil refining catalyst,Efficient Waste Oil Refining Catalyst,QY OC on Alibaba.com
it seems to me we have to work on temperature managing devices to keep catalyst temperature at suitable level. maybe heat it electrically at process start, and cool it (by air blow??) at working along.Originally posted by Jetijs View PostIn my opinion this process with catalysts in the refluctor works about 50% using the catalytic effect and other 50% using good olt thermal cracking. We found that better results are obtained if the refluctor is cooled and not allowed to get past 150 degree celsius, becouse othervise the heavy fractions get past the refluctor. Also, the bottom of the refluctor should be made in a cone shape, like this \/, well not so sharp, just to make a little funnel so that the condensed fuel in the refluctor can pour back in the reactor. When I used a hydraulic press to press out the plastic that filled the refluctor in one of my mishaps, I bent it inside a bit by accident, like this ^ and the results after that were very bad, I got waxes again and semliquid fuel, more like a souce. This is because some of the condensed liquid in the refluctor could not get back to the reactor. When I fixed this problem, fuel came out good and clear again. Clay can be used at least for 5 runs and maybe even more, I just never tried it for more times
it would be interesting to see how refineries control that in fractionning towers. as the product they get at every level hightly depends of the temperature.
please let me add another reflexion :
what is the difference between a gazeous state and a liquid state of an oil ? it seems to me it's the distance between the atoms. are the link between the atoms are longuer ? maybe this also helps for the cracking ? possibly it make molecules easier to be chopped ? or easily availiable ?
Comment
-
to silver
I agree with your concern. If the process is not under vacuum, we should find a easily to obtain gaz to fill the system before operation to avoid this risk.
fill with a gaz and push the air out.
-water steam ? fine but the device has to be at steam temp otherwise it will condensate very quick, and has the side effect of introducing water in the system
-butane cooking gaz ? fine but better to use uncombustible gaz : would be safer
-co2 : hard to get a can of it
-argon : expensive
-azote : expensive
-refrigeration gaz : expensive and dissolve bad things into oil
-any other idea ?
Originally posted by silver View PostHello jetijs,
I planned everything perfect and feeling frightened about one thing. The shell is heated and the gases will reach the condenser. How there will be no explosion when the oxygen in the condenser meets the gases. And when you open the pipe to releive the oil from the condensor how it is sure that the outside air will not leak inside again causing explosion.
Comment
-
keith20mm, awesome build
Islander, car catalyst converter will only work with PP and PE as these materials don't really take any carbon with them when in gaseous phase, all the carbon is left in the reactor and the rest of the system is clean. Another thing if the catalytioc reaction leaves atomic carbon behind, then this catalyst converter could cogg very fast as it has very fine air tunnels in it that cog easily. This will not work well with WMO as it will cog at the first run. Maybe if you break the catalyst into small pieces and use those in the refluctor?
It is worth to try, only those catalyst converters are expensive compared to other catalyst types.It's better to wear off by working than to rust by doing nothing.
Comment
-
Car exhaust catalyst
It is a very good idea adapting standards to these setups Islander, I thought about the car exhaust catalyst too but they are designed to avoid the hydrocarbons to pass through them...
I am afraid it would collapse soon...
Originally posted by islander View PostNow we could try to use a car exhaust catalyst ! It's made for gasses and ready to connect and use.
Does anyone think expanded clay pellets used in gardening would be useful as a catalyst?
Hydroton expanded clay pellets hydroponic growing medium 50 liter bag
Comment
-
islander, with PE I never had any carbon in the produced fuel, maybe a bit of white misty stuff that settles down over time and can be easily filtered off. That stuff is wax. Even when the wax problem was not solved, no carbon was ever foun in the produced fuel/wax. When heated, it always became clear.It's better to wear off by working than to rust by doing nothing.
Comment
-
Much information you desire are contained in Ozmotech's patent: PROCESS AND PLANT FOR CONVERSION OF WASTE MATERIAL TO LIQUID FUELOriginally posted by islander View PostHello
your study is highly interesting !
it seems to me we have to work on temperature managing devices to keep catalyst temperature at suitable level. maybe heat it electrically at process start, and cool it (by air blow??) at working along.
it would be interesting to see how refineries control that in fractionning towers. as the product they get at every level hightly depends of the temperature.
please let me add another reflexion :
what is the difference between a gazeous state and a liquid state of an oil ? it seems to me it's the distance between the atoms. are the link between the atoms are longuer ? maybe this also helps for the cracking ? possibly it make molecules easier to be chopped ? or easily availiable ?
The fraction tower contains several temperature-controlled zones, 60C, 20C, and 8C, condensers within the tower, cooler condensers closer to the tower upper end.
Under each condenser coil is placed a tray on which the condensed liquid is collected, and the tray has a drain line takeoff through the outer wall of the tower tank.
Non-condense gas goes out top of tower.
I would surmise that one could make a common tower section in a stackable form, and just stack these on top of each other, with condensers heated as needed for various fractions, using small water heaters with thermostats and small circulating pumps.
Comment
-
We think that you can't really tell anything from the weight only. We distilled the lighter fuel samples and found that at 150 degree celsius it leaves about 80% of diesel behind, so the gasoline to diesel ratio even in these lighter fuels in our case was 20:80. On the other hand that fuel was very light color, but had more of a yellow/orange color. We have not tried distilling our bets yellow/green samples, they could have higher gasoline content.Originally posted by imakebiodiesel View PostYou dont say which sample weighed 770 gms per litre. A litre of pure gasoline weighs 740, kerosene 780 and diesel 840. So a sample weighing 770 will be mostly lighter fractions, gasoline and kerosene with very little diesel. To produce a heavier diesel type fuel it might be better to crack at a higher temp, say 400, and reduce the waxing by adding a pour point depressant. What do you think.It's better to wear off by working than to rust by doing nothing.
Comment
-
I take your point Jetijs, we cant rely on the specific gravities of commercial fuel as a guide. Fuel from waste plastic is obviously just different. and distillation is the correct way to analyse it. I have just finished my reflux column and am waiting for my clay pellets to dry. i shall try a batch by your method in a few days.
Comment
Comment