A word pf caution re the Sulphur content. DO NOT use sulpher rich fuels in modern Vehicles that have Catalytic converters in the Exhaust system. Many modern Diesels now have thee for No2 emmision control and Sulphur simply blocks them up and renders them useless.
Announcement
Collapse
No announcement yet.
How to turn plastic waste into diesel fuel cheaply
Collapse
X
-
I reviewed the report again. I note the sample color is described as black and opaque. Color would be one measure of distillation quality. The black would be carbon/soot most of which should remain as retort residue.Originally posted by rozier56 View PostDiesel report on some truck engine oil that we converted.
WOULD APPRECIATE YOUR COMMENTS!
NO CATALYST USED.
In my plant distilling and cracking WMO to diesel, the retort temperatures target is 380°C - 420°C range. Going above darkened the condensate stream proportionally. At the same time reflux temperature target at 350°C produces diesel weight fuel averaging .85 SG. Lowering the reflux target to 320°C last time resulted in reduced SG of .835
Rozier, where are your TC located? Can you draw them in?
Comment
-
rozier56
Tanks for your comments.
Need to point a few points of info.
Wheels the inner basket shown in the distillation column is actually filled with baffle materials, ie. mild steel bolts and packed tightly providing good surface area for distillation.
The reduced flow pipe that Excaliber has from the distillation to the condenser i believe will slow down the gas outflow escaping from the distillation to the condenser.Because the system is open ended, it wont necessary cause a back pressure but must influence flow rate to the condenser. If one looks at most moonshine stills depicted you will see they have a similar design.I believe this allows better condenser operation. The other option is to go with bigger/longer condenser. I am going to try Excaliber style first to see the result.
Excaliber my temp probe on the distillation column is on top of the right bend before going to condenser.We ran that oil run at 360*c at distillation.
I also found out that the sample sumitted was actually taken from my plant sample point prior to filtration, hence high impurities.{not to smart}
I have noted that when we run plastic or oil there is always what i call, small black carbon carry over. We now filter out that at end of process. Due you also have this happen in your machine?
Thanks Folks.
Comment
-
Yes. And to be honest, I don't know why apart from assuming it is because of the viscosity of the Hydrocarbon being vaporized. My Plant processes ABS Plastics (don't try this anyone, the stuff is toxic, corrosive and difficult to process) and ABS is full of Plasticizers. Mostly Clay. It is silky smooth fine clay particles and it all comes through the process to the end fuel. The only way to remove it is centrifuge, because the particles are so fine, they go straight through filters, or at the least, plug them instantly. So it is not always because of boil over. I have a feeling it could be because a Fuel/oil molecule is long enough that it can partly wrap itself around very fine particles. And we are talking particles fine enough to go right through filters, so they are at least less than 5micron in size. A Centrifuge will take particles down to 0.1 of a micron. But it is a long slow process of cycling the Fuel till it is clean.Originally posted by rozier56 View PostI have noted that when we run plastic or oil there is always what i call, small black carbon carry over. We now filter out that at end of process. Due you also have this happen in your machine?
Thanks Folks.
Comment
-
Rozier, I would put another TC here....
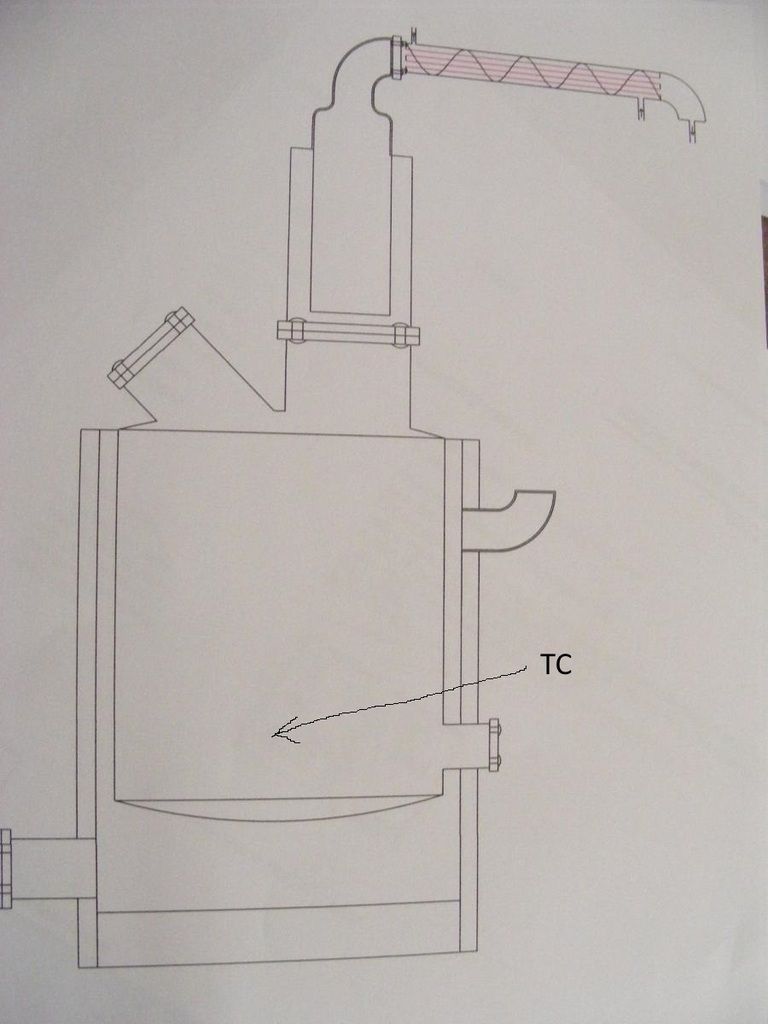
This TC would be connected to a temperature controller that keeps retort temperature within a predetermined range.
I think that even in an industrial distillation plant there'd be some degree of particulate carry over. Ever notice how the 2nd condenser distillate is always cleaner and clearer? The object is to gently, gently heat the feedstock, holding it within a tight temperature range. Avoid hurrying/bullying the boiling process because it leads to a poorly distilled product. It's the operators job to find the retort temperature point that gives good flow but excludes excessive particulate carry over. The rule is: higher temperature is not always better. It's a balance between the 2 parameters.
Keep talking and sharing ideas..
Comment
-
Yes and that also fits my my thoughts on the Molecular length grabbing particulates and carrying them through. The second pot will have far shorter chains.Originally posted by Excalibur View PostEver notice how the 2nd condenser distillate is always cleaner and clearer?
By the way, Viscosity can be linked to chain length, although in theory, it is a lot more complex than that.
Comment
-
Observing my plants' distillate flow in the sight glass have taught me a lot. In the early stages of a run the condensate is perfectly clear. As retort temperature increases the color turns to beautiful yellow amber. Push temperature higher has the color change to shades of red. Higher still has the color go to dirty brown opaque.
The trick seems to be, keep the flow coming at amber to lighter red. However the temptation is to crank up the heat because you want faster flow. Faster will work and even yield the correct weight but color will suffer.
In the moonshine industry they have what's called a doubler. Such a vessel is used to improve purity. In short the product stream is condensed and immediately re-evaporated. A series of doublers can improve quality in stages. I had thought about using similar technique but the downside is the cost of additional heat. Pondering on the subject I thought my vapor stream exiting the reflux could be quenched just enough to liquefy. Immediately it would be reheated to say 325°C subsequently re-evap'ing. This would be a form of double distilling...
Comment
-
My pyrolysis is essentially how Wheels described his, a series of pots, which function, like Excalibur's description of the "doubler" in the distillery. I also have a pot for each cut as well.
However, the downside, as Excalibur pointed out, is more heaters, more heat, but a lot more control, because all of my heaters are electric, which gives greater control.
The result is my product ranges from clear to amber.I have been running various blends of waste oils and unleaded gasoline in a 1983 Chevy G-20 van with a 6.2L diesel V-8 engine, with a Stanadyne Rotary DB2 IP since Feb, 2007. I have started the engine with no difficulty and no block heater on an 80/20 (WVO/gas) blend down to 0F (-18c). I have found that by blending as little as 15% gasoline in the summer, and as much as 50% in the winter, my engine starts and runs as if it was running on diesel fuel.
Comment
-
Yes the only way to do it in my opinion. The more control results in a more precise output range of Hydrocarbon.Originally posted by Beyond Biodiesel View PostMy more heaters, more heat, but a lot more control, because all of my heaters are electric, which gives greater control.
The result is my product ranges from clear to amber.
I have electric heating on all my pots, but so far have no used them. This is only because from the Catalyst onwards, my Plant takes a slightly different track than everyone else. Although my catalyst cracks, the main reason for it to be there is for removal of a specific chemical that is used in all Ewaste plastics as a fire retardant. If that is not removed, it causes all sorts of corrosive issues and is very toxic. Plus it retards combustion of the Fuel in the Burner. It will do the same in an engine and for such a reason, I have not gone too far down the path of using my Fuel in an engine just yet. I have done it to test if it works, but I need to do a great deal more work in that area to understand long term issues that may or may not arise. Anyway....
back to the story. So my Catalyst is heated to a very high temperature to initiate the conversion process and then the Vapor stream needs to be cooled dramatically before reaching the First Pot. But what I have found is that the Vapor arrives at the first pot at a suitable temperature and so I do not need to heat it again. A bonus because it save energy heating the Pot. That captures any heavy fractions and the vapor is allowed to flow to the second pot, where I am trying to rapidly get it down to a much cooler temperature before any "solvent" ranges flow on to the Chilled pot. If I allow to much heat to flow on into the Chilled pot, then the chiller can not cope and it gets too warm and I end up with a very light fuel floating on top of the bubbler water.
I am currently working on a new feed design and that will allow the continual feeding of the plant along with being able to process multiple plastic types separately, yet simultaneously and each type can have a particular process applied to meet their individual need. But it will result in a wider ranging hydrocarbon output that will need greater effort in cracking and containing.
Comment
-
Yes, if I had a need for additional cuts/fractions, a series of pots is the way I'd be doing it and of course, do it while the vapor/condensate stream is hot.Originally posted by Beyond Biodiesel View PostMy pyrolysis is essentially how Wheels described his, a series of pots, which function, like Excalibur's description of the "doubler" in the distillery. I also have a pot for each cut as well.
However, the downside, as Excalibur pointed out, is more heaters, more heat, but a lot more control, because all of my heaters are electric, which gives greater control.
The result is my product ranges from clear to amber.
The thought about greater control with electric heaters highlights the challenges faced with diesel fired heating. I regard the type of heat generated by electric elements as "soft heat", quite unlike the fierce, ferocious heat from a high powered burner. Recent revamp of my plant was an attempt to reduce any overheating of the steel retort vessel and refractory. In addition my burner is adjustable for heat output from nil to 1000°C+. I can match exactly the heat requirement of retort contents. For example once the retort reaches optimum temperature as shown by output flow in the sight glass, then flame temperature may as well be reduced to a figure slightly above retort.
Waste oil feedstocks should be regarded as likely having multiple fractions. Lighter fractions should be boiled off before raising temperature to the boiling point of the next lightest fraction.
BB's description of fuel produced from clear to amber indicates it was made at a rate it was happy to be distilled at, unhurried and allowed to proceed at the pace it wanted. Clearly we cannot apply unlimited heat and expect all to be well. Best to apply heat to closely match the requirements of the liquid we are distilling.
Comment
-
Originally posted by Excalibur View PostYes, if I had a need for additional cuts/fractions, a series of pots is the way I'd be doing it and of course, do it while the vapor/condensate stream is hot...
BB's description of fuel produced from clear to amber indicates it was made at a rate it was happy to be distilled at, unhurried and allowed to proceed at the pace it wanted. Clearly we cannot apply unlimited heat and expect all to be well. Best to apply heat to closely match the requirements of the liquid we are distilling.I agree with wheels here. I find multiple pots equals more fractions, and more precise fractionations, which reduces boil-over, which produces a better product. The fractions can then be all too easily recombined later to produce whatever fuel I want, ie. diesel fuel. And, so far I have found no reason to boil-off light fractions up to 50% of the fuel blendOriginally posted by wheels View PostYes the only way to do it in my opinion. The more control results in a more precise output range of Hydrocarbon.
As we have discussed earlier in this thread, cracking halogenated hydrocarbons is full of problems, especially toxic vapors that the operator is exposed to. I personally believe that halogenated hydrocarbons are so toxic that there should be a law passed that requires all halogenated hydrocarbons be returned to the manufacturer for disposal or recycling. And, we DIY petroleum crackers and refiners should stick to non-halogenated hydrocarbons.Originally posted by wheels View PostI have electric heating on all my pots, but so far have no used them. This is only because from the Catalyst onwards, my Plant takes a slightly different track than everyone else. Although my catalyst cracks, the main reason for it to be there is for removal of a specific chemical that is used in all Ewaste plastics as a fire retardant. If that is not removed, it causes all sorts of corrosive issues and is very toxic. Plus it retards combustion of the Fuel in the Burner. It will do the same in an engine and for such a reason, I have not gone too far down the path of using my Fuel in an engine just yet. I have done it to test if it works, but I need to do a great deal more work in that area to understand long term issues that may or may not arise. Anyway....
back to the story. So my Catalyst is heated to a very high temperature to initiate the conversion process and then the Vapor stream needs to be cooled dramatically before reaching the First Pot. But what I have found is that the Vapor arrives at the first pot at a suitable temperature and so I do not need to heat it again. A bonus because it save energy heating the Pot. That captures any heavy fractions and the vapor is allowed to flow to the second pot, where I am trying to rapidly get it down to a much cooler temperature before any "solvent" ranges flow on to the Chilled pot. If I allow to much heat to flow on into the Chilled pot, then the chiller can not cope and it gets too warm and I end up with a very light fuel floating on top of the bubbler water.
I am currently working on a new feed design and that will allow the continual feeding of the plant along with being able to process multiple plastic types separately, yet simultaneously and each type can have a particular process applied to meet their individual need. But it will result in a wider ranging hydrocarbon output that will need greater effort in cracking and containing.Last edited by Beyond Biodiesel; 07-19-2015, 01:05 PM.I have been running various blends of waste oils and unleaded gasoline in a 1983 Chevy G-20 van with a 6.2L diesel V-8 engine, with a Stanadyne Rotary DB2 IP since Feb, 2007. I have started the engine with no difficulty and no block heater on an 80/20 (WVO/gas) blend down to 0F (-18c). I have found that by blending as little as 15% gasoline in the summer, and as much as 50% in the winter, my engine starts and runs as if it was running on diesel fuel.
Comment
-
Absolutely and a reason why I have always warned members to stay away from anything other than the two relatively safe plastics being PP and PE.Originally posted by Beyond Biodiesel View PostAnd, we DIY petroleum crackers and refiners should stick to non-halogenated hydrocarbons.
It's also one reason why I have never discussed with any great detail of what I do, being the destruction of these toxic plastics.
But besides the Toxic issues, none of these plastics are economical to process for fuels anyway. They are very low yielding and they require specialized equipment and design and far to much post processing to remove and dispose of the Toxic products.
Comment
-
Hello to everyone
We stop working due to bureaucratic obstacles. video link-related tests are below. If you're curious about something, I'm around.
1. home reactor https://www.youtube.com/watch?v=VlJZCBD8nt0
2. only paraffin https://www.youtube.com/watch?v=1xaq_oKcIqs
https://www.youtube.com/watch?v=kOscVDOy96A
3. finished https://www.youtube.com/watch?v=1Khn29RY1WU
Comment
-
Yes that is the single biggest issue for most of us trying to do this commercially. Especially now that many countries are working on trying to reduce Co2 levels. They would rather see Plastic buried in Landfills. Here in NZ, we have to have a thing called Resource Consent. It can cost tens of thousands and often in the Millions of Dollars and sometimes as long as 10yrs to work through the process and have the Bureaucrats tick all the boxes.Originally posted by kedigen View PostHello to everyone
We stop working due to bureaucratic obstacles.
Comment

Comment