Something you should know .... is that most of the elements ( amino acid / dna / rna etc.... ) has left Chiral 
Untitled Document

Untitled Document
Additionally, because of the geometry of the way carbon works as the backbone of organic molecules, it is possible to create molecules that are right- or left-handed. This ability is called chirality. It should be noted that not all organic molecules are chiral: some can be symmetrical. The chiral molecule, however, has properties that are crucial to the working of the chemistry of life. As far as life on Earth is concerned, all normal chiral molecules associated with life processes are left-handed.
In terms of basic chemistry, left- and right- chiral molecules are identical, but their behavior can be vastly different. This is because the chemistry of life depends on the ability of molecules to form atomic bonds at several points simultaneously, to create new compounds. The molecules, however, are three-dimensional structures and to make the bonds work they must be turned round and fitted, much as a jigsaw puzzle would be put together. The consequence is that molecules must be the correct shape, in order to form the required bonding, as well as being chemically correct.
Players of the computer jigsaw puzzle game, Tetris, will appreciate the problem. Some pieces are handed - they are chiral - and however they are turned around and manipulated, they will not fit into the available slots. In fact Tetris would soon become incredibly and boringly easy, if the chiral pieces were not present.
So, in terms of bio-chemistry, you can see that it is impossible for the "wrong-handed" molecule to be used in a particular process, even if it is chemically correct. This characteristic of some organic molecules is key in the processes of life, particularly in replication, such as in the behavior of DNA.
Let us now return to the humble carbon atom and think about its role when considering life elsewhere, off planet Earth. The unique characteristics of carbon give it a versatility and functionality like no other atom in the periodic table. These are why carbon is the basis of life here. Additionally, carbon is only beaten in abundance by hydrogen, helium and oxygen, at least within the Solar System. It is ten times more abundant than silicon and a million times more abundant than boron, which have also been suggested as alternative base elements for living molecules.
In terms of basic chemistry, left- and right- chiral molecules are identical, but their behavior can be vastly different. This is because the chemistry of life depends on the ability of molecules to form atomic bonds at several points simultaneously, to create new compounds. The molecules, however, are three-dimensional structures and to make the bonds work they must be turned round and fitted, much as a jigsaw puzzle would be put together. The consequence is that molecules must be the correct shape, in order to form the required bonding, as well as being chemically correct.
Players of the computer jigsaw puzzle game, Tetris, will appreciate the problem. Some pieces are handed - they are chiral - and however they are turned around and manipulated, they will not fit into the available slots. In fact Tetris would soon become incredibly and boringly easy, if the chiral pieces were not present.
So, in terms of bio-chemistry, you can see that it is impossible for the "wrong-handed" molecule to be used in a particular process, even if it is chemically correct. This characteristic of some organic molecules is key in the processes of life, particularly in replication, such as in the behavior of DNA.
Let us now return to the humble carbon atom and think about its role when considering life elsewhere, off planet Earth. The unique characteristics of carbon give it a versatility and functionality like no other atom in the periodic table. These are why carbon is the basis of life here. Additionally, carbon is only beaten in abundance by hydrogen, helium and oxygen, at least within the Solar System. It is ten times more abundant than silicon and a million times more abundant than boron, which have also been suggested as alternative base elements for living molecules.






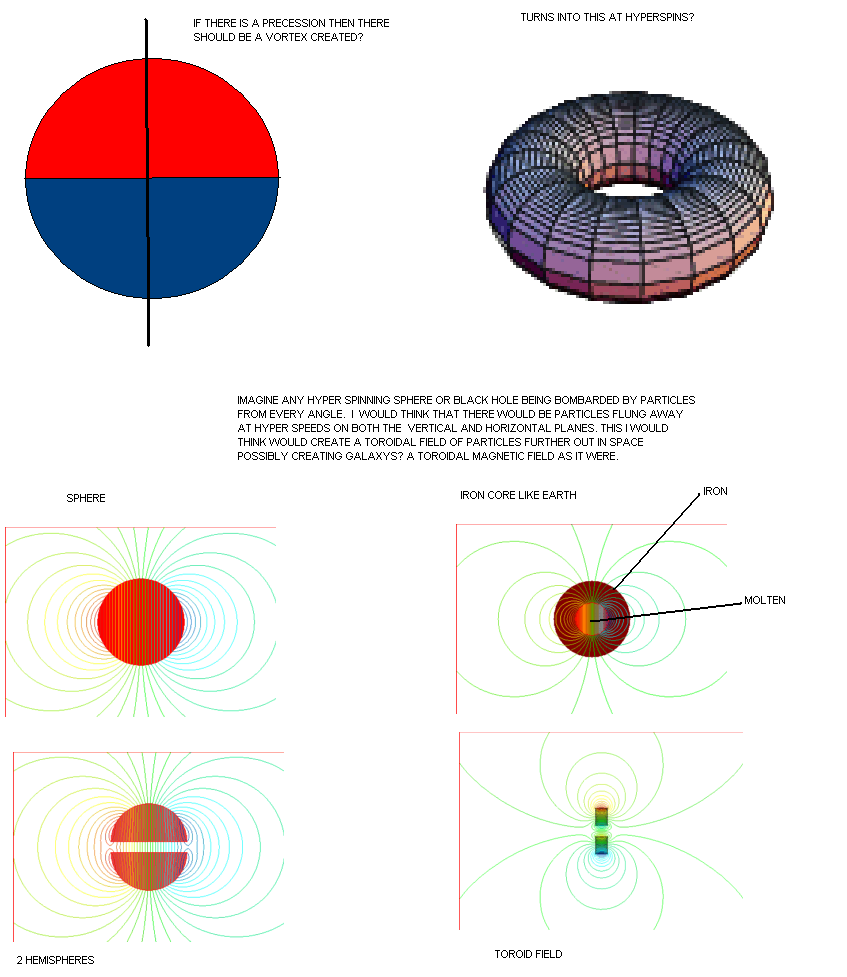

Comment