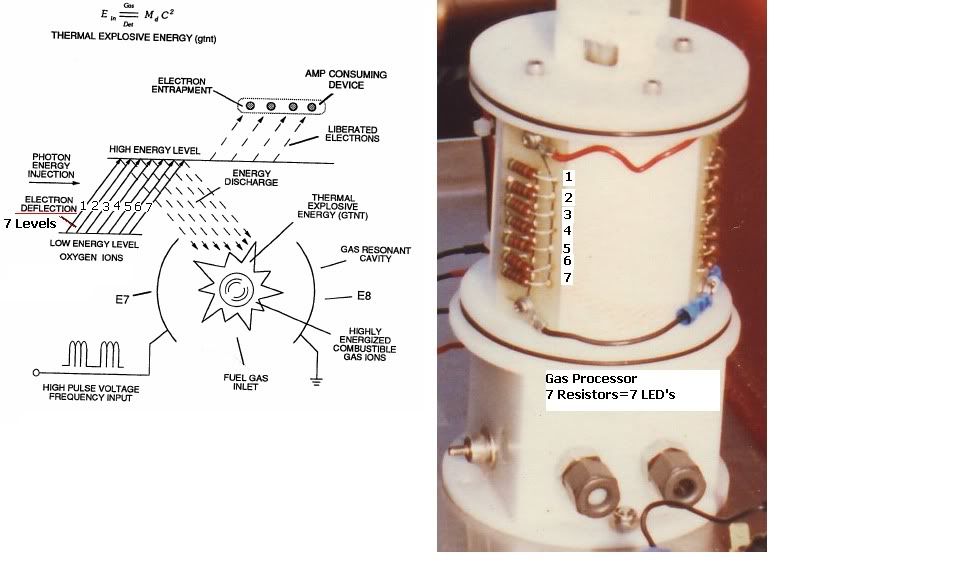
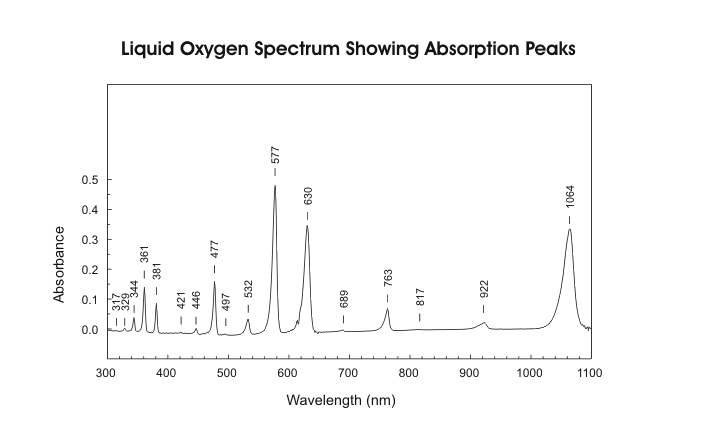
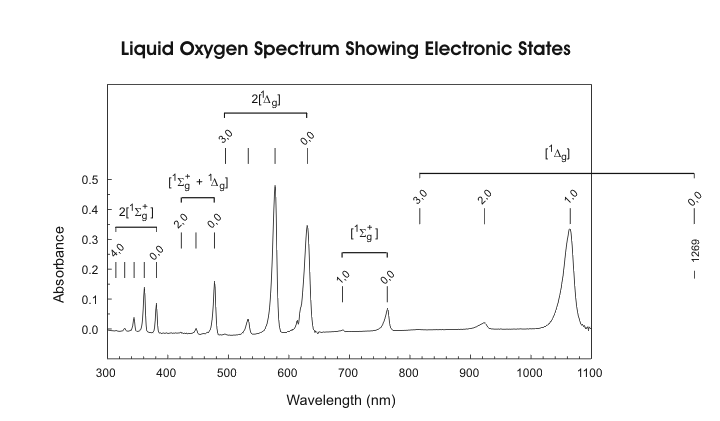
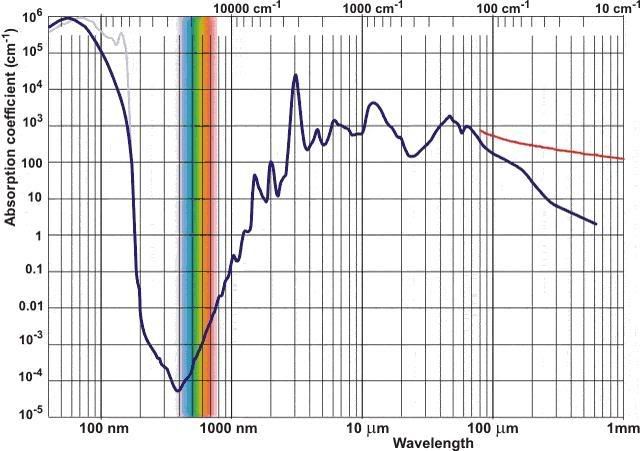
I have found a site that sells 760nm LED's for $2.20 U.S. a piece, they have a minimum order of 50 though. They also sell all the other wavelengths I think we need. But again, still working on understanding the wavelengths required. I think the chart above helps out quite a bit. Need to study Oxygen absorption wavelengths more, but I think I see what they are now, just need to do more studying to know for sure.
Molded type LED
Price List
That site I posted also talks about the aurora lights here:
Oxygen Physical Properties
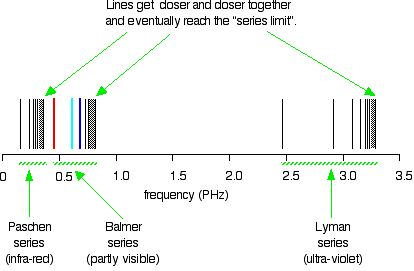
Near the bottom of the page it talks about Atomic Oxygen and the emissions being 558nm and 630nm. It seems if I remember right though that the emission spectrum is different from the absorption spectrum. It looks like the 630nm is the same for both though.
Molded type LED
Price List
That site I posted also talks about the aurora lights here:
Oxygen Physical Properties
Near the bottom of the page it talks about Atomic Oxygen and the emissions being 558nm and 630nm. It seems if I remember right though that the emission spectrum is different from the absorption spectrum. It looks like the 630nm is the same for both though.


Comment