Originally posted by Aaron
View Post
Announcement
Collapse
No announcement yet.
Ionization & Water Fuel
Collapse
X
-
-
Meyers and Nitrogen
Yes, I see importance of nitrogen now.
I think Meyers intentionally misled people through all of his documents
for protection.Sincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
I have every written that .. but only for protect the system.... because in fact when peoples und as work isn't complicated replace that .. but.. in all case N(OH)20 is only first stage..Originally posted by Aaron View PostYes, I see importance of nitrogen now.
I think Meyers intentionally misled people through all of his documents
for protection.
Comment
-
Meyer and process
espacenet — Bibliographic data
In that patent, he shows ambient air going in mixing with hydrogen.
It is burned and the "non combustible" which he only shows oxygen
but of course there is going to be nitrogen, that oxygen/hydrogen
gets routed back to mix with hydrogen and get moved through again.
Adjusting oxy/nit volume back to hydrogen can probably increase
or decrease flame temp. Less oxy/nit, the more "browns gas" type
flame and with more oxy/nit, the more thermal energy we get out.
Do you see the same on this patent?
-------------------------------------------------------
So if first stage is just the production of the "nitrogen hydroxide."
It gets compressed/heated and homogenized...heat could
help do more ionization (thermionic emission) and then
the piston moves down after TDC creating vacuum pulling
apart hydrogen from oxygen and nitrogen from hydrogen?
Then there is plasma, which separates h2 into h1 and nitrogen
absorbs electrons and so does maybe some of the oxygen
to prevent h2 from joining oxygen and forming water after
combusion?Sincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
Plasma is needed because inside mixture that you create you don't have only N(OH)2 but also "conductive" water doplets inside and from combustion you need do not obtain some water vapour from exaust gas but similarry to standard engine, of course without COx products and no more NOxOriginally posted by Aaron View Postespacenet — Bibliographic data
In that patent, he shows ambient air going in mixing with hydrogen.
It is burned and the "non combustible" which he only shows oxygen
but of course there is going to be nitrogen, that oxygen/hydrogen
gets routed back to mix with hydrogen and get moved through again.
Adjusting oxy/nit volume back to hydrogen can probably increase
or decrease flame temp. Less oxy/nit, the more "browns gas" type
flame and with more oxy/nit, the more thermal energy we get out.
Do you see the same on this patent?
-------------------------------------------------------
So if first stage is just the production of the "nitrogen hydroxide."
It gets compressed/heated and homogenized...heat could
help do more ionization (thermionic emission) and then
the piston moves down after TDC creating vacuum pulling
apart hydrogen from oxygen and nitrogen from hydrogen?
Then there is plasma, which separates h2 into h1 and nitrogen
absorbs electrons and so does maybe some of the oxygen
to prevent h2 from joining oxygen and forming water after
combusion? Last edited by tutanka; 01-30-2010, 09:47 AM.
Last edited by tutanka; 01-30-2010, 09:47 AM.
Comment
-
@Tutanka
It is very interesting!
I think this will stimulate a new trend in the water fuel world.
There are some "nitrogen hydroxy" websites but none are really
exploring chemistry beyond the idea of "nitrogen hydroxy".
Well, I think the answer is here in this thread thanks to your help!
I can try some experiments in a few months.
There are many others now with plasma ignition and hho cell experience
that are in a position to just apply a few more things! Everyone that has
failed with just HHO I hope will see a different perspective.
This is powerfully exciting! Sincerely,
Sincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
Aaron I agree with you but the very important point is that YOU DON'T NEED TO PRODUCE MORE HYDROGEN FROM WATER .This little thread created in few days contains more true informations, people can work on that and I'm sure reach the success in no more time.. of course .. there are some others points to clarify, for example the true function of exaust gas, but important is to take the right way..Originally posted by Aaron View PostIt is very interesting!
I think this will stimulate a new trend in the water fuel world.
There are some "nitrogen hydroxy" websites but none are really
exploring chemistry beyond the idea of "nitrogen hydroxy".
Well, I think the answer is here in this thread thanks to your help!
I can try some experiments in a few months.
There are many others now with plasma ignition and hho cell experience
that are in a position to just apply a few more things! Everyone that has
failed with just HHO I hope will see a different perspective.
This is powerfully exciting!
 Last edited by tutanka; 01-29-2010, 04:39 PM.
Last edited by tutanka; 01-29-2010, 04:39 PM.
Comment
-
 Wow, just freaking wow...I am not a chemist or physicist, but I know when I see something of great significance. This thread has got to rank at the top of the list.
Wow, just freaking wow...I am not a chemist or physicist, but I know when I see something of great significance. This thread has got to rank at the top of the list.
All that was missing was the plasma plug...
They always said for the thing to work you needed to create a lightning ball inside the combustion chamber and I never knew what they meant by that.
You know, I am not sure Meyers even knew exactly how his stuff worked. So I am not certain he was trying to mislead anyone. He just did it in a different way. There is always going to be more than one way to get from A to B.
I watched a video of when he and his partner introduced steam into the engine for the first time and if you listen closely he was excited about the water vapor keeping the engine's temperature under control.
This stuff burns real hot without it...hotter than the surface of the sun if I remember correctly.
great work guys,
Murlin
Comment
-
exhaust purpose?
Is exhaust after combustion primarily n2, o2 and h2?Originally posted by tutanka View Posttrue function of exaust gas
It is hot exhaust I would imagine. Heat helps to cause ionization by
"thermionic emission".
If exhaust is hot and it leaves the engine, it helps cool the engine. lol
I guess that is obvious but just listing what I can think of off the top
of my head.
If that exhaust is reused, we have h2 separated from o already meaning
that it is easier to combust over and over if it is recycled with less
energy needed each time?
I guess as you mentioned that some of the combustion byproduct is
some nox and moisture comparable to gasoline combustion except none
of the carbon-based emissions.Sincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
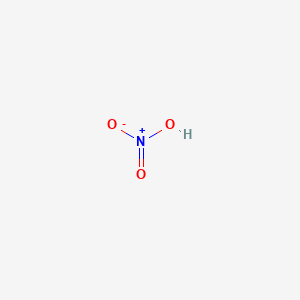
N(OH)2O = Nitrogen Hydroxide Oxide
N(OH)2O = Nitrogen Hydroxide Oxide
NHO3 is same thing?
Is this gas a form of gaseous nitric acid?
-----------------------------------------------------------
Nitric Acid - PubChem Public Chemical Database
Nitric Acid - Compound Summary (CID 944)
Nitric acid (HNO3). A colorless liquid that is used in the manufacture of inorganic and organic nitrates and nitro compounds for fertilizers, dye intermediates, explosives, and many different organic chemicals. Continued exposure to vapor may cause chronic bronchitis; chemical pneumonitis may occur.
Explosive Agents - Substances that are energetically unstable and can produce a sudden expansion of the material, called an explosion, which is accompanied by heat, pressure and noise. Other things which have been described as explosive that are not included here are explosive action of laser heating, human performance, sudden epidemiological outbreaks, or fast cell growth.
- Substances that are energetically unstable and can produce a sudden expansion of the material, called an explosion, which is accompanied by heat, pressure and noise. Other things which have been described as explosive that are not included here are explosive action of laser heating, human performance, sudden epidemiological outbreaks, or fast cell growth.
-----------------
I think I posted this before but:
Wiley InterScience :: Session Cookies
Experimental Detection of the H2NO3 Radical
Fulvio Cacace, Prof. Dr. *, Giulia de Petris, Prof. Dr. *, Anna Troiani, Dr.Dipartimento di Studi di Chimica e Tecnologia delle Sostanze Biologicamente Attive, Università di Roma La Sapienza
La Sapienza , P.le A. Moro 5, 00185 Roma, Italy, Fax: (+39)06-49-913-602
, P.le A. Moro 5, 00185 Roma, Italy, Fax: (+39)06-49-913-602
email: Fulvio Cacace (fulvio.cacace@uniroma1.it)*Correspondence to Fulvio Cacace, Dipartimento di Studi di Chimica e Tecnologia delle Sostanze Biologicamente Attive, Università di Roma La Sapienza
La Sapienza , P.le A. Moro 5, 00185 Roma, Italy, Fax: (+39)06-49-913-602
, P.le A. Moro 5, 00185 Roma, Italy, Fax: (+39)06-49-913-602
*Correspondence to Giulia de Petris, Dipartimento di Studi di Chimica e Tecnologia delle Sostanze Biologicamente Attive, Università di Roma La Sapienza
La Sapienza , P.le A. Moro 5, 00185 Roma, Italy, Fax: (+39)06-49-913-602
, P.le A. Moro 5, 00185 Roma, Italy, Fax: (+39)06-49-913-602
setDOI("ADOI=10.1002/cphc.200300849")Keywordsinorganic radicals • gas-phase chemistry • mass spectrometry • nitric acid • short-lived intermediatesAbstractIt's in the air. The authors detected by neutralization-reionization mass spectrometry the nitrogen hydroxide oxide 3, which is relevant to atmospheric chemistry and a key intermediate in the NO32- reduction, as a gaseous radical species with a lifetime of 1 s. The charged precursor utilized was the high-energy isomer 2 from the protonation of HNO3, whereas the more stable isomer 1 dissociates upon neutralization.
s. The charged precursor utilized was the high-energy isomer 2 from the protonation of HNO3, whereas the more stable isomer 1 dissociates upon neutralization.Received: 20 May 2003Digital Object Identifier (DOI)
10.1002/cphc.200300849 About DOISincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
N(OH)2O = Nitrogen Hydroxide Oxide
Found this quote online:
"Nitrogen is completely non-flammable, that does not prevent it from rapidly bonding with Hydrogen when separated from it's O2 Counterpart. Nitrogen Hydroxide, N(OH)2O, is one of the most volatile incendiary gasses and bonds when hydrogen, oxygen, and nitrogen are present as individual molecules."Last edited by Aaron; 01-29-2010, 05:54 PM.Sincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
Meyer also reuses exhaust
Meyer shows in multiple patents the re-ducting of exhaust or cell output back
to be mixed with newly formed hho.
espacenet — Bibliographic data
Abstract of EP 0111574 (A1)
System and apparatus for the controlled intermixing of hydrogen volatile gas with non-combustible gasses in a combustion system. The system utilizes a hydrogen generator (10) for developing a controlled output of hydrogen and oxygen gasses and non-volatile gasses such as nitrogen. The hydrogen gas with the attendant gasses and added gasses are fed via a line (5) (9) to an air intake system (20) in a controlled ratio. The combined gasses after intermixing are fed to a combustion chamber (30) wherein the mixture is ignited. The exhaust gasses of the combustion chamber (30) are returned in a closed loop arrangement to the mixing chamber (40) as non-volatile gasses to control the velocity and temperature of the volatile hydrogen gas.

This link is the pic...Last edited by Aaron; 01-29-2010, 06:42 PM.Sincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
Exaust gas are recycled inside to engine because these react as initially inside the cell forming N(OH)2...Originally posted by Aaron View PostMeyer shows in multiple patents the re-ducting of exhaust or cell output back
to be mixed with newly formed hho.
espacenet — Bibliographic data
Abstract of EP 0111574 (A1)
System and apparatus for the controlled intermixing of hydrogen volatile gas with non-combustible gasses in a combustion system. The system utilizes a hydrogen generator (10) for developing a controlled output of hydrogen and oxygen gasses and non-volatile gasses such as nitrogen. The hydrogen gas with the attendant gasses and added gasses are fed via a line (5) (9) to an air intake system (20) in a controlled ratio. The combined gasses after intermixing are fed to a combustion chamber (30) wherein the mixture is ignited. The exhaust gasses of the combustion chamber (30) are returned in a closed loop arrangement to the mixing chamber (40) as non-volatile gasses to control the velocity and temperature of the volatile hydrogen gas.

This link is the pic...Last edited by tutanka; 01-31-2010, 05:58 PM.
Comment
-
I guess that answer my previous confusionOriginally posted by Aaron View Post
Found this quote online:
"Nitrogen is completely non-flammable, that does not prevent it from rapidly bonding with Hydrogen when separated from it's O2 Counterpart. Nitrogen Hydroxide, N(OH)2O, is one of the most volatile incendiary gasses and bonds when hydrogen, oxygen, and nitrogen are present as individual molecules." . I guess plasma is a requirement to make this NH3 since it need to be in high temperature. Maybe the preheating do not need to be plasma and can be done with only exhaust gas temperature.
. I guess plasma is a requirement to make this NH3 since it need to be in high temperature. Maybe the preheating do not need to be plasma and can be done with only exhaust gas temperature.
Comment
-
That circuit generate unipolar voltage wave, is dated 1980. I think Meyer use similar of that.. use every an capacitor, in fact Plasma circuit is the next generation and is IMPORTANT part of system because wthout that you CAN'T generate THERMAL EXPLOSIVE ENERGY,in all case you don't need only N2+ + 2OH- = N(OH)2.Originally posted by sucahyo View PostI guess that answer my previous confusion . I guess plasma is a requirement to make this NH3 since it need to be in high temperature. Maybe the preheating do not need to be plasma and can be done with only exhaust gas temperature.
. I guess plasma is a requirement to make this NH3 since it need to be in high temperature. Maybe the preheating do not need to be plasma and can be done with only exhaust gas temperature.
Is important understand that point, hydrogen CAN'T mix with air outside from engine as air/gasoline. However now peoples understand that Meyer don't want generate oxygen destabilized with gas processor but other electrochemical mixture Last edited by tutanka; 04-20-2010, 08:01 PM.
Last edited by tutanka; 04-20-2010, 08:01 PM.
Comment

Comment