ammonia reaction?
Tutanka posted this on Feb 01 in response to sucahyo's post. I mentioned
ammonia earlier than that but no comment.
Catalyst is HHO and ionized air is important to combust ammonia (that
other molecule). There may also be air that is not ionized.
Ammonia is what is created here - this is what Stan Meyer was doing.
---------------------------------------------------------------------
Quote:
Originally Posted by sucahyo
I think catalyst and air mixture is also important if the gas is NH3.
I think NH3 should be the goal, maybe deserve a new thread but let see the result of italian team first.
BTW, I feel like I repeating someone else word, someone that claim to have understood it. Sorry that I forget your name and where you post them.........
TUTANKA: Good illumination.. Regards
Regards
----------------------------------------------------------------------
Tutanka post on 1/28...
Quote:
Originally Posted by sucahyo
Not before it explode. I am talking about the event after the explosion. The endothermic reaction part do not start before reaching very high temperature.
What you describe sound more like implosion after small explosion. I am talking about temperature after the combustion chamber reach plasma temperature.
I think the exothermic reaction(NH3/ammonia creation) will happen along with the first fire and help catalyst the burning. After the temperature reach the endothermic reaction requirement (HNO3 creation) it will made a bigger bang and sudden temperature drop because of big energy requirement, along with greener gas output. Only theory though.
Tutanka: Hello,
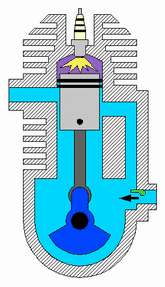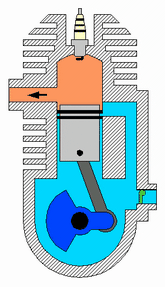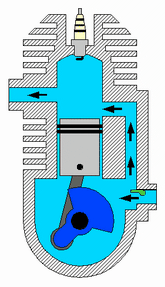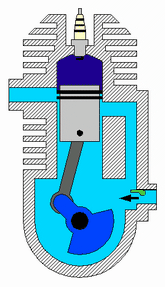
This is my consideration after test, thermal explosive energy is reached ONLY using an catalist because you forget that you use only water that not burn usually. In fact new fuel mixture must be unstable, water is stable so it does not react. Object of that project is create unstable mixture for reach an fastly exothermic reaction because you want use that into an engine that run at some rpm. We have called that "micronic fuel mixture" that react only with nascent plug and plasma. About Meyer.. I'm sure that inside injector of meyer is created low amount of catalist, not all water droplets are transformed inside..
-----------------------------------------------------------------------
The creation of ammonia is an exothermic reaction. And normally happens
in the presence of a catalyst.
So are there MULTIPLE catalysts here?
Is the ammonia created in the compression chamber when compressed
and heated and in contact with a metal catalyst of tungsten or some other
metal on the plug?
Then once the ammonia is created, the plasma ignition ignites, burning
the HHO, which is a catalyst to combust the ammonia?
-----------------------------------------------------------------------
In either case, the "nitrogen hydroxide" is an ammonia fuel.
Tutanka posted this on Feb 01 in response to sucahyo's post. I mentioned
ammonia earlier than that but no comment.
Catalyst is HHO and ionized air is important to combust ammonia (that
other molecule). There may also be air that is not ionized.
Ammonia is what is created here - this is what Stan Meyer was doing.
---------------------------------------------------------------------
Quote:
Originally Posted by sucahyo

I think catalyst and air mixture is also important if the gas is NH3.
I think NH3 should be the goal, maybe deserve a new thread but let see the result of italian team first.
BTW, I feel like I repeating someone else word, someone that claim to have understood it. Sorry that I forget your name and where you post them.........
TUTANKA: Good illumination..
 Regards
Regards----------------------------------------------------------------------
Tutanka post on 1/28...
Quote:
Originally Posted by sucahyo

Not before it explode. I am talking about the event after the explosion. The endothermic reaction part do not start before reaching very high temperature.
What you describe sound more like implosion after small explosion. I am talking about temperature after the combustion chamber reach plasma temperature.
I think the exothermic reaction(NH3/ammonia creation) will happen along with the first fire and help catalyst the burning. After the temperature reach the endothermic reaction requirement (HNO3 creation) it will made a bigger bang and sudden temperature drop because of big energy requirement, along with greener gas output. Only theory though.
Tutanka: Hello,
This is my consideration after test, thermal explosive energy is reached ONLY using an catalist because you forget that you use only water that not burn usually. In fact new fuel mixture must be unstable, water is stable so it does not react. Object of that project is create unstable mixture for reach an fastly exothermic reaction because you want use that into an engine that run at some rpm. We have called that "micronic fuel mixture" that react only with nascent plug and plasma. About Meyer.. I'm sure that inside injector of meyer is created low amount of catalist, not all water droplets are transformed inside..
-----------------------------------------------------------------------
The creation of ammonia is an exothermic reaction. And normally happens
in the presence of a catalyst.
So are there MULTIPLE catalysts here?
Is the ammonia created in the compression chamber when compressed
and heated and in contact with a metal catalyst of tungsten or some other
metal on the plug?
Then once the ammonia is created, the plasma ignition ignites, burning
the HHO, which is a catalyst to combust the ammonia?
-----------------------------------------------------------------------
In either case, the "nitrogen hydroxide" is an ammonia fuel.
 2NH3(g)
2NH3(g) 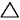 H = -92.4 kJ mol-1
H = -92.4 kJ mol-1  .
.
 My air processor used special circuit double HV output 30+30Kv, the main output is AC, the other (grid) negative DC. With that I obtain positive/negative ions..
My air processor used special circuit double HV output 30+30Kv, the main output is AC, the other (grid) negative DC. With that I obtain positive/negative ions.. 


Comment