Originally posted by pengrove
Announcement
Collapse
No announcement yet.
Ionization & Water Fuel
Collapse
X
-
Can you explain the process? Are you ionizing the the steam with the nitrogen at the same time? If so you must also have a magnetic field to keep the oxygen from recombining with the hydrogen. Is 18kv DC enough? I have a static generating power supply here. I am not so sure about the electrode configuration at this point. Your discharge tube design would be interesting to see. How much pressure and what kind of temperatures are we talking about here? The discharge tube would need to handle these high pressures and temperatures but also let a magnetic field through.
Contact me on Skype if you would please. I would like to have a conversation. My user name is divclone. Last edited by pengrove; 04-22-2010, 07:19 PM.
Last edited by pengrove; 04-22-2010, 07:19 PM.
Comment
-
For last time.. I can't write complete process but I can help you to open your mind. The story start from old experimenters like ArchieBlue with the creation of nitrous hydroxide.. In fact nitrous hydroxide don't exist, is just an name for identified new molecule.. if you see ArchieBlue patent him mix air inside electrolytic cell for obtain nitrous hydroxide; that some years ago.. today appear that can be possible that nitrous hydroxide can be ammonia NH3 but as you know we use water that chemically is H-H-O, if 1+1=2 the same is for atoms and molecules.. In all case for run the process you need also energy or for understand better e- .. you can use low voltage and high current or viceversa.. for that you can use standard electrolytic cell like Bob Boyce or other device.. I suggest to see all days the first diagram system of meyer posted from Sucahyo..Originally posted by pengrove View PostCan you explain the process? Are you ionizing the the steam with the nitrogen at the same time? If so you must also have a magnetic field to keep the oxygen from recombining with the hydrogen. Is 18kv DC enough? I have a static generating power supply here. I am not so sure about the electrode configuration at this point. Your discharge tube design would be interesting to see. How much pressure and what kind of temperatures are we talking about here? The discharge tube would need to handle these high pressures and temperatures but also let a magnetic field through.
Contact me on Skype if you would please. I would like to have a conversation. My user name is divclone.
 Last edited by tutanka; 04-22-2010, 09:56 PM.
Last edited by tutanka; 04-22-2010, 09:56 PM.
Comment
-
Boyce and Archieblue both use Electrolyte. I though that Electrolyte would keep the reaction from working properly?Originally posted by tutanka View PostFor last time.. I can't write complete process but I can help you to open your mind. The story start from old experimenters like ArchieBlue with the creation of nitrous hydroxide.. In fact nitrous hydroxide don't exist, is just an name for identified new molecule.. if you see ArchieBlue patent him mix air inside electrolytic cell for obtain nitrous hydroxide; that some years ago.. today appear that can be possible that nitrous hydroxide can be ammonia NH3 but as you know we use water that chemically is H-H-O, if 1+1=2 the same is for atoms and molecules.. In all case for run the process you need also energy or for understand better e- .. you can use low voltage and high current or viceversa.. for that you can use standard electrolytic cell like Bob Boyce or other device.. I suggest to see all days the first diagram system of meyer posted from Sucahyo..
Comment
-
Searching the net on power consumption for corona discharge systems and found this paper:Originally posted by tutanka View PostSorry but for me don't have sense.. I suggest you to study pulsed corona discharge.. Regards
PowerPoint Presentation - Energy-efficient transient plasma ignition
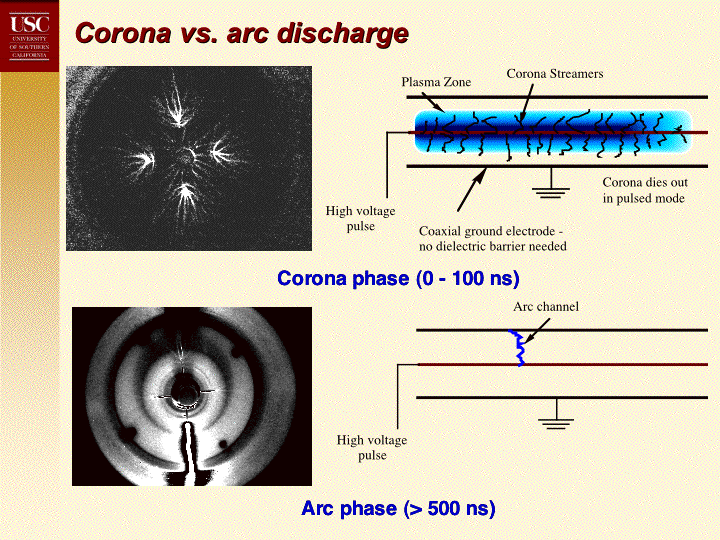
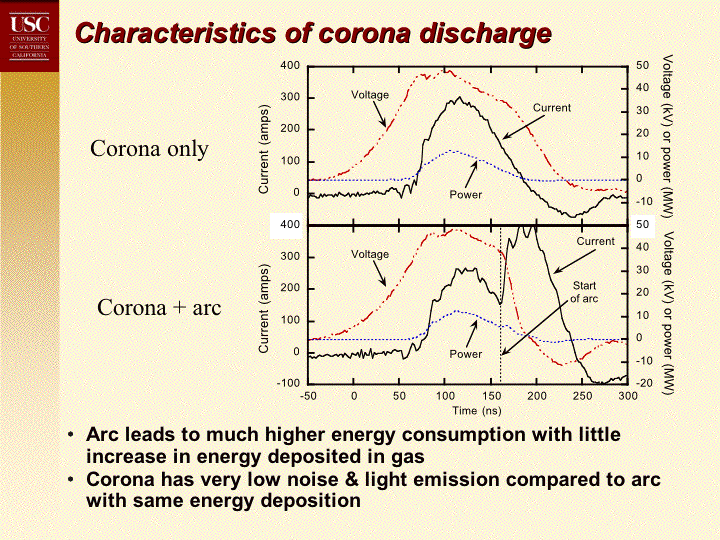
Regards, Mike R.
Comment
-
There is a reason why H2opower is not here and we like that. Them who wants to follow h2opower can do so in other forums, there is also a reason why he do not post here.Originally posted by chasson321 View PostI think everyone should read this:
Tim
This tread is no longer limited to Meyer, so this eliminates that post - the energy content and balenced equetions is still needed to calculate production rate and fuel concumption - but I think I can speak for most here when I rant you on the fact that no one besides h2opower is "limited" to use the exact same amount water / hdyrogen avalible. This is only a "fact" if a similar VW engine is used etc ... phu, I am so tired of reading this stuff sometimes.
H2Opower refuses to change and see facta that is presented here over and over again and everyone can do energy calculations. The only reason h2opower is used here is becouse you do not understand the physical reaction that are involved to form nitrogne tri-hydrogen. Energy concumption is NOT the only situation you need to solve, you are also dealing with fuel handeling inside you're engine ... Try to compress hydrogen gas over time in an piston engine and see what happens, that is the only way you will change you're direction and research angle.
Liqiud fuel is needed during compression to maintane the fuel within the cylinders if we look at a "normal" piston engine - forget all other engines becouse that is not what this is about, it is about a fuel conversion for 99% of existing car models and the engine version used is almost always the Otto Cycle static pressure port engine.
"But we have gas engines" - uhm ... LPG and CNG - ya, but they are still larger molecules then H2 ...
Normal hydrogen filled cars use cryogenic hydrogen = liquid prior to combustion - weird huh. aka it is only in a gasues state during expantion - Behold the truth -
- Behold the truth -
Comment
-
ThanksOriginally posted by vrand View PostSearching the net on power consumption for corona discharge systems and found this paper:
PowerPoint Presentation - Energy-efficient transient plasma ignition . Lol, that article is what I was trying to find yesterday.
. Lol, that article is what I was trying to find yesterday.
I found this instead:
It seems silent discharge mentioned in 100 years old book is the corona produced by sharp point not the arc. However, my experiment show that whenever corona starting to appear, it produce loud noise. I can make video because the spark jam my keyboard....5.3 Ionization System
An effective ionization plate must be capable of interacting with the ionized particles that it creates without the complications of uncontrolled arcing. The method of ionization for this study is to pass the uniformly dispersed seed particles though a high voltage DC electric field, known as field ionization. For this case, a strong electric field forcibly extracts an electron from an atom. By charge exchange ionization, electrons are exchanged with another atom through the outer valence shells. The dimensions of the electrodes are dictated by the minimum separation distance required to prevent arcing [89]. Thin electrodes also tend to produce more of a corona discharge effect due to the concentration of charge on sharp surfaces. Using these principles, an ionization actuation plate was constructed with five electrodes (two positive, three ground) using approximately a one inch spacing between each. A 20 kVDC Glassman power supply is used to generate the electric field. For the maximum voltage, the electrode spacing is close to the minimum requirement to prevent arcing (roughly 1 mm of separation per kV for air under normal conditions). Since the seeded air has an elevated electrical conductivity, arcing is prone to occur. However, it is hypothesized that the air flow will somewhat reduce the tendency of arcing between the electrodes. Simple experiments conducted thus far using the ionization plate showed that an arc can be effectively "blown out" by a low-speed flow. Therefore, it is plausible that DC ionization of seeded air using a glow discharge can still take place without arcing although there may be a limited range of operation between conditions that suppress arcing while generating an appreciable amount of ionized particles.
I notice flaw in his chemistry at the first post of his theory thread. He won't answer my question about it, I don't think I will answer that. It is about how he calculate energy of water in and water out.Originally posted by chasson321 View PostI think everyone should read this:Tim
In here we talk more about water+ambient air in and something else out before combustion chamber.Last edited by sucahyo; 04-23-2010, 03:42 AM.
Comment
-
Hello guys
I think i found something...
Meyer said
" and the water de-energized will go to the atmosphere and be again energized by the incoming sun rays...
What if he was having at his exhaust:
OH-
or H3O+
or something like that
What do you think is the energy needed to break this into h2 again if it is already at 600°C?
Do you know that at this temperature there is laser energy everywhere, IR energy... ?
What if the magnetic fields could change the attraction forces witch holds the molecule together?
5000 gauss magnetic field a neodymium magnet can provide easily...even more and it would lose its magnetism only if get too hot...
Meyer used a heat sink...
Just some food for your thoughts
Some Quantum Chemistry would make lot of good to you guys
also
Atomic and Molecular Physics
Regards
Comment
-
On the second thought I think I should correct some mistakes in h2opower post, because it seems h2opower never read link that I post and also this also to remind everyone here not to use high temperature, if NH3 is the goal:
1. Heat is not the only ionization energy we can use. We can also use vacuum, light and electricity.
2. Everyone use water as input. Any energy being used to convert them to something else (hydrogen or NH3) must be included in calculation.
3. To avoid mistakes and to give clear view, every calculation must be done in mole. A conversion from/to mass should only be done at the beginning or at the end of calculation. I got lost tracking the mass refer to what mole since it is never mentioned clearly.
4. Temperature required for NH3 synthesis is not 500°C:
Haber process - Wikipedia, the free encyclopedia
High temperature is required by the catalyst to work, not by the NH3 reaction.As the temperature increases, the equilibrium is shifted and hence, the amount of product drops dramatically according to the Van't Hoff equation. Thus one might suppose that a low temperature is to be used and some other means to increase rate. However, the catalyst itself requires a temperature of at least 400 °C to be efficient.
This graph bellow means there will be more NH3 at lower temperature:

They don't use spark at NH3 plant for safety I guess...
BTW, after reading the post, I still don't understand what is the relation between limiting reagent and the death of nitrogen theory.
Comment
-
You need electron reduction - that is the first half of the reaction. You need to replace the valance electrons with certain atoms....Originally posted by pengrove View PostCorona discharge produces Ozone but do you think that it also produces Positive Nitrogen Ions?
Com on you guy's - is there no one that actually see all of this by now????- Behold the truth -
Comment
-
analyze the old references
You may ignore this as you have with my other points, but I'll give you andOriginally posted by chasson321 View PostI think everyone should read this
H2OPower the benefit of the doubt for the second, third or fourth time...
Between the both of you, analyze the nearly 100 year old references that I
posted in this thread showing the production of NH3 from high voltage,
air and water. Each study referenced accomplishes NH3 production, literally
on demand in relatively low temperature and low pressure and each study
does it in a slightly different way. It's all there in the books, you cannot
dispute it.
According to your analysis, none of those results are possible and your
analysis does not take into account other energy releasing mechanisms
giving further evidence of a misunderstanding of the entire process.
It is as if you think nitrogen goes Ninja style through an ionizer untouched
and unnoticed by high voltage and/or photon energy and/or magnetic
fields and slips through as a fairly non-reactive N2. There is something
not just wrong with that perspective, but astoundingly wrong.
The process described here is perhaps cousins with that old science and
is not the exact same process, but certainly is related to the basic
aspects of the system. Water, air, HV, etc...
Take one reference at a time, analyze it and post the analysis. That way,
we can all see first of all the real chemistry knowledge of you/h2opower
and in addition to that, it will show the level of sincerity and authenticity.
Don't lump all the references under one analysis either. Give an analysis
of each one, if you're able.
If all of that is automatically discounted as being irrelevant, flawed, etc...
then all I can say is that any further posts from you in this thread will
simply be deleted because it will be obvious that you are not interested in
a two-way conversation and are simply pushing your anti-nitrogen
propaganda. And don't try to claim censorship as many wishful thinkers
like to claim because it won't be true. You have a chance to address the
valid points that are posted in this thread and 100% of the time so far,
you and h2opower ignored them. Last chance to demonstrate honesty, Tim.
Also not interested in seeing it pointed out that these studies aren't
producing high quantities of NH3 - the point is... obviously NH3 on
demand production is not only possible, it is practically required to happen!
Volume of nh3 production in those old tests is irrelevant - the fact that
it can and does happen IS what is relevant, truthful and real.
And while you're at it, post your analysis of something relevant like the
two Lateral Science articles on Langmuir.
---------------------
p.s. Here is one more reference that was posted by Schpankme in another
thread - you can include an analysis of this one - now it is included in this
thread so nobody can say they never saw it:
http://www.energeticforum.com/91409-post14.html - (Schpankme)
Many trying to replicate hydrogen through Electrolysis would be extremely happy utilizing the low-tech methods dating back to the civil war and described in this book:
The Chemistry and Manufacture of Hydrogen
by P. Litherland Teed
164 pages 5.5x8.5 inches [size]
Knowledge Publications
has one of the best books ever written on Hydrogen, this is the first printing of The Chemistry and Manufacture of Hydrogen since 1919! With the republication of this book and others like it we are realizing the most fundamental purpose for producing written records: the preservation and rediscovery of knowledge. - Schpankme
----------
http://www.energeticforum.com/91414-post16.html
Thanks!
Here is a pdf of the 1919 version - IT'S FREE.
http://www.archive.org/download/chem...00teedrich.pdf - (me)
----------
http://www.energeticforum.com/91504-post29.html - (me)
I looked through about 75% of the book that Schpankme posted
last night - very simple straight forward explanations - even nh3 reference
from just nitrogen + hydrogen + silent electric discharge
page 26 - "With Nitrogen* Donkin has shown that when a
mixture of hydrogen and nitrogen is subjected to the
silent electric discharge, a partial union of the two gases
takes place, with the formation of ammonia :
N2 + 3H2 = 2NH3 .
However, this reaction could in no way be regarded as
commercial, as the quantity of ammonia produced after
the gases have long been subjected to the silent electric
discharge is only just sufficient to be identified by the
most delicate means.
Recent investigations have, however, shown that if
the two gases are mixed and subjected to very great
pressure (1800 Ib. per sq. inch) in the presence of a
catalytic agent, union to an appreciable extent takes
place. This process, which is now being used on a
CHEMICAL PROPERTIES 27
commercial scale in Germany, is known as the Haber
process, but few details as to the method of operation
are available. In the earlier stages of the working of
this process the catalytic agent was probably osmium,
but it is considered doubtful if this is still being employed. "
Yes, not sufficient for commercial production - however,
super ionized nitrogen is NOT accounted for amongst a few
other parts of the full reaction - but I'm happy to see the
reference and further proof of NH3 on demand production.Last edited by Aaron; 04-23-2010, 05:07 AM.Sincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
nh3
This point might set a world's record for the most blatant hints on oneOriginally posted by Oneminde View PostYou need electron reduction - that is the first half of the reaction. You need to replace the valance electrons with certain atoms....
Com on you guy's - is there no one that actually see all of this by now????
single point. I hope nobody feels games are being played because there are
so many straight up honest hints as to this one single piece and it keeps
flying right on by. Every time someone is about to nail it and I mention that
it is about to be nailed, it is like a distraction comes from the collective to
get everyone close off on some other direction. Actually, I think that is
more true than most would believe.
Just to let everyone know, anyone that knows this one point based on a
successful school-of-thought, pretty much had to figure it out themselves.
Others WAY before Meyer figured it out, others shortly before and around
the same time Meyer did figured it out and others in a more recent times
figured it out.
Anyway, the post I'm replying to here just spelled it out a little too clearly,
to my surprise. lolSincerely,
Aaron Murakami
Books & Videos https://emediapress.com
Conference http://energyscienceconference.com
RPX & MWO http://vril.io
Comment
-
Guy's look at what Aaron wrote then look at this tread ...
"Every time someone is about to nail it and I mention that
it is about to be nailed, it is like a distraction comes from the collective to
get everyone close off on some other direction. Actually, I think that is
more true than most would believe."
Yes, that is probably more true then moste want to know. It is actually scary to see how close some of the members here are and just fly by not noticing that they actually did see the solution, makes one think if there is a retarded gen jumping the tread. And yes, my post should - SHOULD - nail it for most of you now. I was wrong when I said "the coin should fall into place soon" sometime ago.. I withdraw that statement now.- Behold the truth -
Comment

Comment