Regular hydrogen being separated out of water has no real power and it takes a lot of it to run an automobile engine. If on the other hand you convert the hydrogen to atomic hydrogen it becomes very powerful and it takes much less of this atomic hydrogen to run an automobile engine and/or whatever else you want to run.
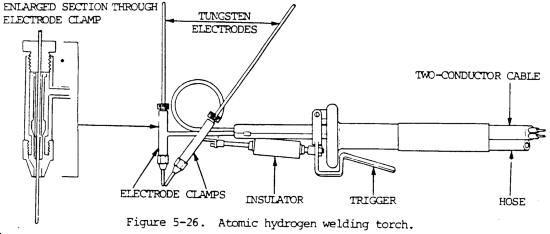
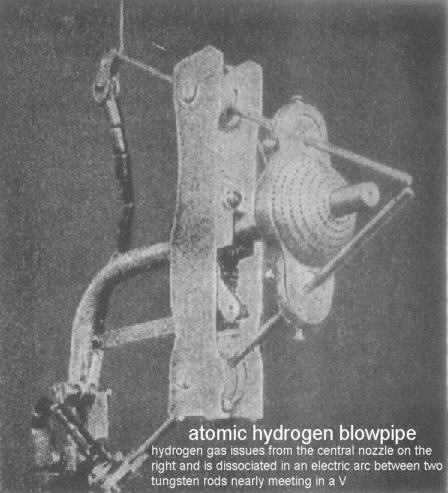
Irving Langmuir invented an atomic hydrogen torch back in 1926 and this is one of the technologies that has been suppressed because the hydrogen being converted in that torch doesn't have to be used for welding but can be used as a fuel.
I saw a thread on the atomic furnace and it has some good information that is applicable here also. I'm not trying to steal anyones thunder from that thread, but I want members to realize that there are other uses for atomic hydrogen such as but not limited to running cars, generators, and so many other things.
Irving Langmuir
Lateral Science - Atomic Hydrogen Blowtorch
Irving Langmuir invented an atomic hydrogen torch back in 1926 and this is one of the technologies that has been suppressed because the hydrogen being converted in that torch doesn't have to be used for welding but can be used as a fuel.
I saw a thread on the atomic furnace and it has some good information that is applicable here also. I'm not trying to steal anyones thunder from that thread, but I want members to realize that there are other uses for atomic hydrogen such as but not limited to running cars, generators, and so many other things.
Irving Langmuir
Lateral Science - Atomic Hydrogen Blowtorch





Comment